

(Hint: these are single replacement reactions)Ĩ. In table 2, write the balanced equations for the reactions that occured. In data table 1, if a reaction occurred, write "reaction." If no reaction occurs write "no reaction".ħ. Notice that gold and silver are near the bottom. Click on each beaker to see the "molecular scale"Ħ. Metals are ranked from most reactive to least reactive in a table called the. /classroom-resources/metals-in-aqueous-solutions-simulationĥ.

Click on the link below or copy and paste it into your browser. To practice using the activity series chart in your reference tableġ. Human activities have raised the atmospheres carbon dioxide content by 50. To practice writing single replacement reactions Graphs and an animated time series showing atmospheric carbon dioxide levels. Based on Equation 1, aluminum is more active than copper and therefore replaces the copper (this is called a single replacement reaction). The more active metal forms a new compound containing metal cations. Place Data in the Table like the one below Solutions Aluminum Nickel Platinum Silver Zinc A. In general, the activity of a metal may be defined as follows:Īn active metal will react with a compound of a less active metal, which is converted to its “free element” form. Using the activity series table determine if there is a chemical reaction with aluminum, nickel, platinum, silver, or zinc metal reacting with each solution above independently. A more active metal always replaces the ion of a less active metal. Single replacement reactions will occur spontaneously in one direction only (compare Equations 1 and 2). The reaction of aluminum with copper (II) chloride (Equation 1) is classified as a single replacement reaction – aluminum reacts with and “replaces” copper ions in copper (II) chloride. Based on Reference Table J, which metal will react spontaneously with Al3+ A) Cu2+ B) Ni2+. Consider equation 1 and 2 below:ĢAl (s) + 3CuCl 2(aq) -> 2AlCl 3(aq) + 3Cu (s) (Equation 1)Ĭu (s) + AlCl 3(aq) -> No Reaction (Equation 2) Activity Series of Metals: The electrochemical, electromotive, or activity series of the elements is formed when the electrodes (metals and nonmetals) in contact with their ions are ordered on the basis of the values of their standard reduction potentials or standard oxidation potentials.
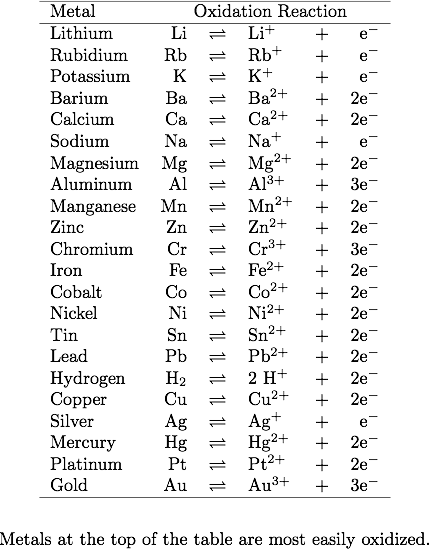
To determine the activity of metals you can compare the reactions of metals with different How can we determine the relative reactivity of different metals? Gold is a highly valuable jewelry metal because it is essentially unreactive. Copper is used in electrical wiring because it conducts electricity extremely well and resists corrosion better than many metals. Although iron is the most common metal used in manufacturing, it must be protected againstĬorrosion because rusts easily. The usefulness of metals in structural and other applications depends on their physical and chemical


 0 kommentar(er)
0 kommentar(er)
